We reviewed all cases of multiple primary melanoma diagnosed at our department over a 32-year period (1987–2019) to better characterize this subgroup of patients and develop a tailored protocol to offer them closer follow-up.
MethodsRetrospective, observational, descriptive study of patients diagnosed with multiple primary melanoma at a tertiary care hospital between January 1987 and March 2019. We collected clinical, epidemiologic, and histologic characteristics of primary and subsequent melanomas and performed a descriptive analysis.
ResultsThirty-one patients (15 men and 16 women) with a median age of 67 years (range, 36–85 years) were included. Second primary melanomas were diagnosed after a median of 2 years (range, 0–4 years). The median number of melanomas per patient was 2 (range, 2–6). Of the 31 patients, 25 had 2 primary melanomas (80%), 4 had 3 melanomas (13%), and 2 patients each had 5 and 6 primary melanomas. Subsequent melanomas were less invasive than the initial primary melanomas. Median Breslow thickness was 1 mm (range, 0.67–4 mm) for the first primary melanoma and 0.5 mm (range, 0.32–2.42 mm) for subsequent melanomas.
ConclusionsSubsequent melanomas are thinner than primary melanomas. We observed an increase in the number of cases of multiple primary melanoma diagnosed in the last 2 years of our study. Our findings highlight the importance of close, long-term follow-up of patients.
Realizamos una revisión de los melanomas múltiples primarios que se han diagnosticado en nuestro servicio a lo largo de los últimos 32 años (1987-2019) con el objetivo de tener mejor caracterizada nuestra población de pacientes con melanoma y poder ofrecerles un seguimiento más estrecho mediante la elaboración de un protocolo de seguimiento personalizado.
MetodologíaEstudio observacional, descriptivo y retrospectivo de los melanomas primarios múltiples diagnosticados en un hospital de tercer nivel entre los años 1987 y marzo de 2019. Se recogieron las características clínicas, epidemiológicas e histológicas de los melanomas primarios, así como de los subsecuentes melanomas y se realizó un análisis descriptivo de las mismas.
ResultadosSe incluyeron 31 pacientes, 15 hombres y 16 mujeres, con una edad mediana de 67 años (intervalo: 36-85 años). La mediana de tiempo transcurrido desde el diagnóstico del primer melanoma primario y del segundo melanoma fue de 2 años (intervalo 0-4 años). La mediana del número de melanomas por paciente fue de 2 (entre 2-6). Del total de 31 pacientes, 24 padecieron 2 melanomas (77%), 4 de ellos 3 melanomas (13%), y dos pacientes presentaron 5 y 6 melanomas primarios respectivamente. Los segundos melanomas primarios o subsecuentes eran menos invasivos comparados con los primeros. La mediana de índices de Breslow fue de 1 mm en los primeros (entre 0,67 y 4 mm) y 0,5 mm (0.32-2,42 mm) en los segundos.
ConclusionesLos melanomas subsecuentes son más finos que los primeros melanomas diagnosticados. Se encontró un aumento de la frecuencia en los dos últimos años de melanomas múltiples primarios. Estos datos resaltan la importancia del seguimiento estrecho y a largo plazo de estos pacientes.
Patients diagnosed with melanoma are at an increased risk of developing additional melanomas. The occurrence of 2 or more melanomas in the same patient is known as multiple primary melanoma (MPM).1
Data from retrospective series indicate that 0.2% to 8.6% of patients with melanoma will develop MPM.2,3 In a series of 283 patients, Slingluff et al.2 estimated a 5% risk for developing a second primary melanoma during a period of 10 years. In another series of 1482 melanoma patients, DiFronzo et al4 found that 4% of patients developed an additional melanoma within 5 years of the initial diagnosis. The most widely accepted 10-year incidence rate for second melanomas is 3.4%.4
Up to 70% of additional melanomas are diagnosed within 2 years of the initial diagnosis.5 Subsequent melanomas are classified as synchronous (diagnosed at the same time as or within 3 months of the first melanoma) or metachronous (diagnosed later).5 Synchronous melanomas account for 30% of cases.2,3
Additional primary melanomas have been shown to be thinner than initial lesions.2–4,6 According to a recent study, the risk of MPM is greatest in elderly white men.6 The most common anatomic location is the head and neck, suggesting a role for sun exposure.6 Patients with MPM have also been found to have a worse prognosis than those with a single melanoma.7
We analyzed the clinical and histologic characteristics of all patients diagnosed with MPM at our hospital during a period of 32 years.
Material and MethodsWe performed a retrospective, observational, descriptive study of patients diagnosed with MPM at a tertiary hospital in Madrid, Spain. We analyzed data from the hospital’s melanoma database and reviewed medical records and pathology reports dating from January 1987 to March 2019.
The study was reviewed by the hospital’s ethics committee, which considered that it involved the analysis of secondary data previously collected during the course of medical care and under the responsibility of the same health care team. The study was conducted in accordance with the principles of the Declaration of Helsinki.
We identified 33 patients with MPM and reviewed their demographic, clinical, and histologic data.
Inclusion CriteriaPatients diagnosed with MPM at our hospital between 1987 and 2019.
Exclusion CriteriaPatients with missing histologic data or information on primary melanomas due to complete histologic regression; patients with noncutaneous (mucosal or uveal) melanoma.
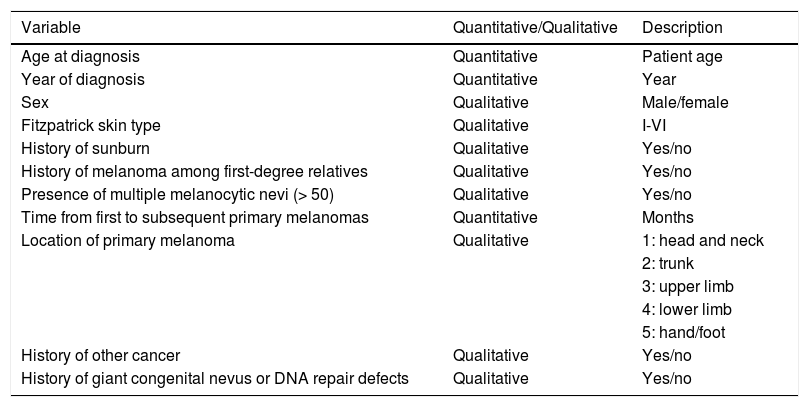
The following epidemiologic and clinical variables were analyzed (Table 1): age at diagnosis, year of diagnosis, sex, Fitzpatrick skin type, episodes of sunburn with macroscopic blisters (reported in the patients' medical records), family history of melanoma, presence of melanocytic nevi (few vs. multiple [> 50]), presence of atypical moles, time from diagnosis of first melanoma to subsequent diagnoses, history of cancer other than melanoma, history of giant congenital nevus and genetic disorders involving a DNA repair defect, and location of first and subsequent primary melanomas. The lesions were grouped into 5 anatomic regions: head and neck, thorax, upper limbs, lower limbs, and hands/feet.
Epidemiologic and Clinical Variables Analyzed.
| Variable | Quantitative/Qualitative | Description |
|---|---|---|
| Age at diagnosis | Quantitative | Patient age |
| Year of diagnosis | Quantitative | Year |
| Sex | Qualitative | Male/female |
| Fitzpatrick skin type | Qualitative | I-VI |
| History of sunburn | Qualitative | Yes/no |
| History of melanoma among first-degree relatives | Qualitative | Yes/no |
| Presence of multiple melanocytic nevi (> 50) | Qualitative | Yes/no |
| Time from first to subsequent primary melanomas | Quantitative | Months |
| Location of primary melanoma | Qualitative | 1: head and neck |
| 2: trunk | ||
| 3: upper limb | ||
| 4: lower limb | ||
| 5: hand/foot | ||
| History of other cancer | Qualitative | Yes/no |
| History of giant congenital nevus or DNA repair defects | Qualitative | Yes/no |
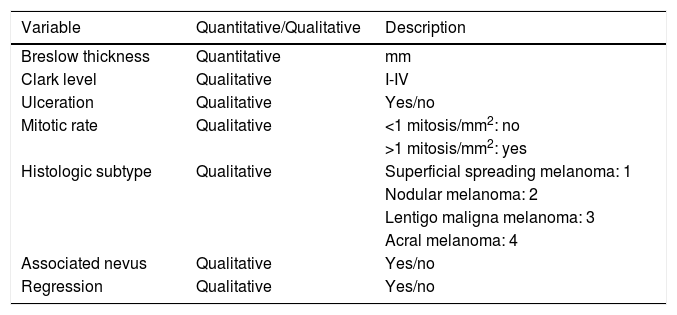
The histologic variables analyzed were Breslow thickness, Clark index, ulceration, number of mitoses, histologic subtype (superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma), and histologic evidence of an associated nevus and regression (Table 2).
Histologic Variables Analyzed.
| Variable | Quantitative/Qualitative | Description |
|---|---|---|
| Breslow thickness | Quantitative | mm |
| Clark level | Qualitative | I-IV |
| Ulceration | Qualitative | Yes/no |
| Mitotic rate | Qualitative | <1 mitosis/mm2: no |
| >1 mitosis/mm2: yes | ||
| Histologic subtype | Qualitative | Superficial spreading melanoma: 1 |
| Nodular melanoma: 2 | ||
| Lentigo maligna melanoma: 3 | ||
| Acral melanoma: 4 | ||
| Associated nevus | Qualitative | Yes/no |
| Regression | Qualitative | Yes/no |
We analyzed the histologic features of initial and subsequent melanomas. The Kaplan Meier method was used to calculate median follow-up times and ranges.
ResultsOf the reference population of 1018 patients diagnosed with melanoma at a tertiary hospital between 1987 and 2019, 33 developed MPM. Two patients were excluded because of insufficient clinical and histologic data (one because the pathology report for the first melanoma was not accessible and the other because the histologic data needed for analysis were not available due to complete histologic regression).
We therefore analyzed 31 patients (16 women [52%] and 15 men [48%] men) with a median age of 67 years (range, 36-85 years).
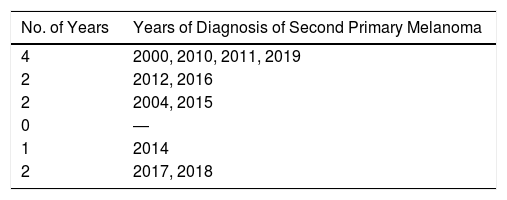
The median time from diagnosis of the first to the second primary melanoma was 2 years (range, 0-12 years). Twelve patients (39%) had synchronous melanomas, while 19 (61%) had metachronous melanomas.
Nineteen patients (61%) were diagnosed with a second melanoma within 3 years of the initial diagnosis.
The median number of melanomas per patient was 2 (range, 2–6). Twenty-five of the 31 patients (80%) had 2 melanomas and 4 (13%) had 3; 2 patients each had 5 and 6 melanomas (Table 3).
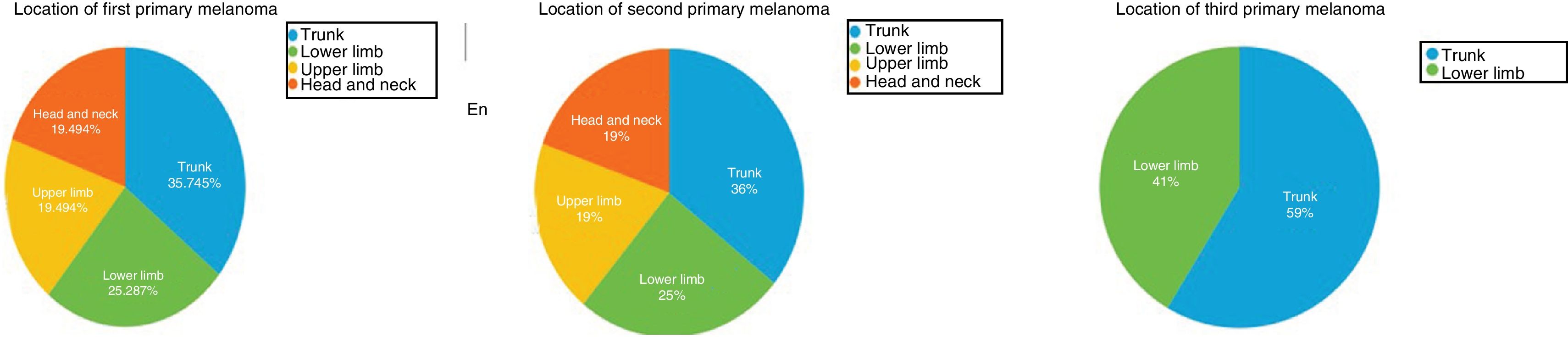
The first primary melanomas were mostly located on the trunk (13/31, 42%) or the lower limbs (8/31, 26%) (Fig. 1). Second and third melanomas were also mostly located on the trunk (11/31 [36%] and 25/31 [80%] respectively). First and second melanomas were located in the same anatomic region in 12 patients (39%).
Atypical moles were reported as being present in 10 patients (32%). Six patients (19%) had a family history of melanoma, but this involved first-degree relatives in just 2 cases (7%). Twenty of the 31 patients (65%) had multiple melanocytic nevi (> 50) according to their medical records.
None of the patients had a history of giant congenital nevus (> 20 cm) or a genetic disorder involving DNA repair defects.
Five patients (16%) had a history of noncutaneous cancer (prostate, breast, sigmoid adenocarcinoma, and papillary thyroid carcinoma).
The most common histologic subtypes were superficial spreading melanoma and lentigo maligna melanoma for both first melanomas (16/28 [58%] and 3/28 [10%], respectively) and second melanomas (17/28 [61%] and 8/28 [29%], respectively). The most common subtype for third melanomas was lentigo maligna melanoma (1/3).
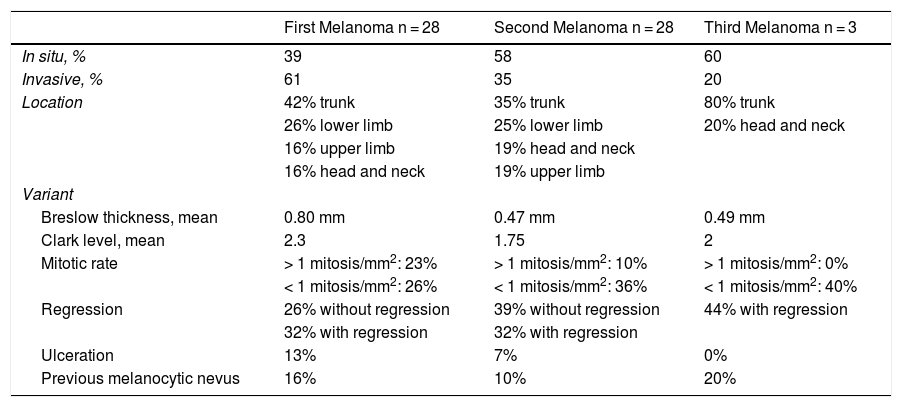
Additional melanomas were less invasive than the initial tumors. The median Breslow thickness for first melanomas was 1 mm (range, 0.67-4 mm) compared with 0.5 mm (range, 0.32-2.42 mm) for second melanomas (Table 4). Overall, 55% of second primary melanomas and 80% of third primary melanomas were thinner than the initial tumors. Thirty-nine percent of first melanomas, 58% of second melanomas, and 60% of third melanomas were in situ.
Histologic Characteristics of Multiple Primary Melanomas.
| First Melanoma n = 28 | Second Melanoma n = 28 | Third Melanoma n = 3 | |
|---|---|---|---|
| In situ, % | 39 | 58 | 60 |
| Invasive, % | 61 | 35 | 20 |
| Location | 42% trunk | 35% trunk | 80% trunk |
| 26% lower limb | 25% lower limb | 20% head and neck | |
| 16% upper limb | 19% head and neck | ||
| 16% head and neck | 19% upper limb | ||
| Variant | |||
| Breslow thickness, mean | 0.80 mm | 0.47 mm | 0.49 mm |
| Clark level, mean | 2.3 | 1.75 | 2 |
| Mitotic rate | > 1 mitosis/mm2: 23% | > 1 mitosis/mm2: 10% | > 1 mitosis/mm2: 0% |
| < 1 mitosis/mm2: 26% | < 1 mitosis/mm2: 36% | < 1 mitosis/mm2: 40% | |
| Regression | 26% without regression | 39% without regression | 44% with regression |
| 32% with regression | 32% with regression | ||
| Ulceration | 13% | 7% | 0% |
| Previous melanocytic nevus | 16% | 10% | 20% |
Most melanomas developed de novo on healthy skin. A previous nevus was described for 16% of first primary melanomas (5/28), 10% of second primary melanomas (3/28), and 20% of third primary melanomas (1/3) (Table 4).
Melanomas were classified into 2 groups according to mitotic rate (< 1 or > 1 mitoses/mm2) (Table 4). Twenty-three percent of first primary melanomas (6/28) had > 1 mitosis/mm2 compared with 10% of second primary melanomas (3/28). None of the third primary melanomas had a mitotic rate of > 1 mitosis/mm2.
Ulceration was present in 13% of first primary melanomas (4/28), 7% of second primary melanomas (2/28), and 0% of third primary melanomas (Table 4).
DiscussionPatients with melanoma have an increased risk of developing additional melanomas. MPM is estimated to affect between 0.2% and 8.6% of patients with melanoma,2,3 although the most widely accepted 10-year incidence rate is 3.4%.4
Although second primary melanomas were uncommon in our series (3%), the risk of additional melanomas in any patient with melanoma must be borne in mind.
Although there is some controversy, it is generally agreed that most melanomas develop de novo and not as a result of malignant transformation of a previous nevus. In our series of MPM, histology showed signs of a previous nevus in 16% of tumors.
There is also some controversy regarding the characteristics of patients with MPM versus those of patients with a single melanoma. An increased risk of melanoma has been described in patients with multiple nevi, with a relative risk of 9.8 reported for patients with over 100 melanocytic nevi. In the case of atypical moles, the relative risk was 3.8 (P = .001) for patients with 1 to 5 moles and 6.3 for those with 6 or more (P = .003).8
In our series, 65% of patients had more than 50 melanocytic nevi while 32% had atypical moles. These rates are consistent with reports to date. Seven percent of patients had a first-degree relative with a history of melanoma.
Sixteen percent of patients in our series (5/31) had a history of noncutaneous cancer, located in the prostate, breast, thyroid, and rectum (2 cases). None of the patients or their relatives had a history of pancreatic cancer.
Sixty-one percent of patients developed a second melanoma within 3 years of the initial diagnosis, but approximately 40% of cases were diagnosed within 3 months. In our review of the literature, we found that 51% to 59% of second primary melanomas were diagnosed within a year; the rate for synchronous melanomas (diagnosed within 3 months of the initial tumor) was approximately 30%.9 Again, our rates for synchronous and metachronous primary melanomas are similar to those described in the literature.2–4 The longest time to diagnosis of the second primary melanoma in our series was 12 years, highlighting the importance of long-term follow-up (> 10 years) and a full clinical examination at each visit.
The most common location for melanomas in our series was the trunk, contrasting with recent reports describing the head and neck.6 These differences could be due to differences in geographic location and consequently sun exposure behaviors. In previous series of MPM, all patients with melanoma of the head and neck developed additional melanomas in the same region, highlighting the potential role of sun exposure in this area.6 In our series, 39% of second primary melanomas developed in the same region as the first melanoma, supporting the field cancerization theory (increased risk of malignant transformation close to the first tumor).10
The patients in our series developed a median of 2 melanomas (range, 2–6). This number is similar to previous reports,10,11 although we believe that number of additional melanomas may be influenced by follow-up time. In addition, patients who undergo melanoma excision will often have other (frequently dysplastic) nevi removed, preventing malignant transformation.
One of the main histologic characteristics described for second primary melanomas to date is that they tend to be thinner than initial melanomas.10,11
Two hypotheses have been proposed to explain why subsequent melanomas have a lower Breslow thickness. The first is that close surveillance facilitates early detection of subsequent melanomas, while the second is that MPM tumors are less aggressive than single tumors.12
Our data support this as 55% of second melanomas and 80% of third melanomas in our series had a lower Breslow thickness than the initial primary tumor. The proportion of melanomas in situ was also higher for subsequent melanomas (58% and 60% for second and third melanomas respectively vs. 39% for first melanomas).
Ulceration was also less common in additional melanomas, although it was still present in 7% of second tumors.
Genetic factors could also contribute to the lower thickness of subsequent melanomas. Functional alterations have been identified in the CDKN2A gene (located on chromosome 9p21) that encodes the protein p16. This mutation has been detected in 8.3% to 15% of patients with MPM.13 Genetic studies were not conducted in patients with MPM at our hospital, although such a project is currently being prepared in conjunction with the oncology department.
Immunomodulatory germline variations were recently described in association with MPM. Ferguson et al.14 studied 41 of these variations in 977 melanomas and detected statistically significant differences suggesting that immune status could have an important role in the development of MPM. The authors suggested that germline genetic variations affecting the immune system might contribute to the development of MPM and stressed the importance of these biomarkers in the follow-up of patients with MPM, who often have to undergo multiple excisions, with both aesthetic and emotional repercussions. The most significant of the 41 alleles analyzed was rs2071304 (referred to by the authors as a minor allele), as patients carrying this allele were 40% less likely to develop MPM. rs2071304 was associated with decreased SPI1 expression, which would reduce sensitivity to self-antigens, causing the immune system to eliminate "micromelanomas" before they become clinically detectable.
Close, long-term follow-up of patients with melanoma is necessary, as the presence of a single primary melanoma increases the risk of a second one, and patients with 2 primary melanomas have an even greater risk of developing a third.
Patients with MPM have been found to have a worse prognosis than those with a single melanoma.7 This was shown in a recent study of 56929 patients in which the authors stressed the importance of closer follow-up due to this worse outlook.15 Their findings have been reproduced in more recent series, including that of Menzies et al.16 We did not analyze survival in our series, although we would like to do so in future studies.
Pastor-Tomás et al.17 recently analyzed risk factors for a second primary melanoma in 1447 patients. They detected an incidence of 3.8% and found that fair skin, a melanocytic count of over 100, and a high number of cherry angiomas were all significant risk factors. The association with cherry angiomas is noteworthy as it has not been previously described and suggests a possible link between sun damage and genetic susceptibility. The authors also found that second primary melanomas were thinner than initial melanomas.
Our study has some limitations, including its retrospective design and small sample size and the fact that we only performed a descriptive analysis. We also did not study survival, although we believe this would be an interesting aspect to consider in future studies. As mentioned, we are planning to perform genetic studies in the near future to investigate the clinical implications of genetic alterations in this setting.
ConclusionsWe have analyzed the clinical and histologic characteristics of 31 patients with MPM. In accordance with previous findings, subsequent melanomas were thinner than initial melanomas. We also observed an increase in the incidence of MPM diagnosed in the last 2 years of our study. Our findings highlight the importance of close, long-term follow-up.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Salgüero Fernández I, Palma Marti L, Nájera Botello L, Roustan Gullón G. Características clínicas e histológicas del melanoma primario múltiple en una serie de 31 pacientes. Actas Dermosifiliogr. 2021;112:52–58.